In a groundbreaking scientific paper recently published, researchers have uncovered a previously understudied regulator of liver tumor progression—the parasympathetic Vagus nerve. The study, titled “The Gut Microbiome Controls Liver Tumors via the Vagus Nerve,” delves into the intricate connections between the gut microbiome, the Vagus nerve, and hepatocellular carcinoma (HCC).
Liver cancer, one of the deadliest forms of cancer, has long been associated with poor outcomes and immunosuppression. The liver’s immune-tolerant environment, constantly exposed to gut-derived antigens, creates an ideal setting for rapid tumor growth and limits the effectiveness of immunotherapy.
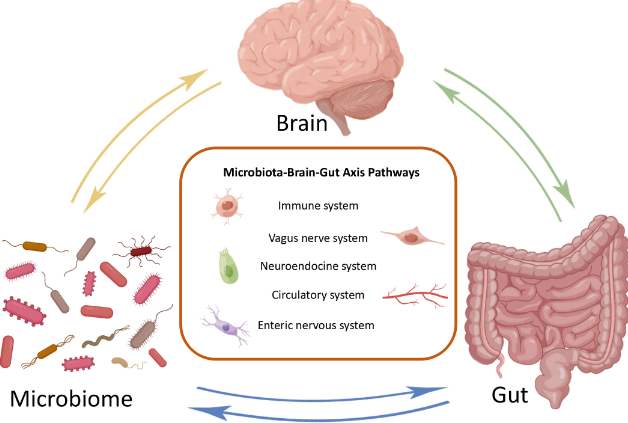
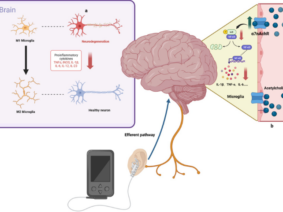
The research, conducted by Bauer et al. (2024), sheds light on the role of nerves in shaping the tumor microenvironment, emphasizing the bidirectional relationship between nerve-tumor signals and neuroimmune circuits. Specifically, the parasympathetic Vagus nerve, a crucial component of the nervous system, was found to modulate visceral organs through acetylcholine (ACh) activity.
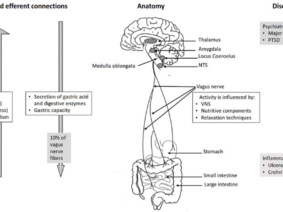
Researchers demonstrated that surgical hepatic branch vagotomy significantly reduced liver tumor burden in mice, while pharmacological enhancement of parasympathetic tone promoted tumor growth. The study further uncovered the intricate interplay between cholinergic neuroimmune interactions and adaptive immunity, with CD8+ T cells playing a pivotal role in liver tumor regulation.
Beyond tumor-specific outcomes, the research highlighted improvements in cancer-associated fatigue and anxiety-like behavior following vagotomy. Microbiota transplantation experiments implicated gut bacteria in influencing behavior and liver anti-tumor immunity, unveiling a dynamic and pharmaceutically targetable vagus-liver axis.
Key Findings:
- Vagus Nerve Regulation: The study highlights the bidirectional vagal activity- vagus to liver and liver to brain—as a crucial factor in liver tumor outcomes. The Vagus nerve’s influence has been implicated in various cancer progressions, including breast, colorectal, gastric, pancreatic, and prostate tumors.
- Cholinergic Anti-Inflammatory Arc: Surgical hepatic branch vagotomy (HV) in mice resulted in reduced liver tumor burden, accompanied by a broad inflammatory response supporting a cholinergic anti-inflammatory arc.
- CD8+ T Cell Involvement: ACh signaling, particularly through the muscarinic ACh receptor CHRM3, was found to dampen CD8+ T cell activity. Depletion of CD8+ T cells abrogated the positive outcomes of HV, and selective deletion of Chrm3 on CD8+ T cells inhibited liver tumor growth.
- Behavioral Improvements and Microbiota Connection: Vagotomy not only impacted tumor-specific outcomes but also led to improvements in cancer-associated fatigue and anxiety-like behavior. The study also explored the crosstalk between microbiota and neuroimmune responses.
For those seeking innovative approaches to liver cancer treatment, this study opens new doors. The findings emphasize the potential of Vagus Nerve Stimulation Devices as a targeted therapy for liver tumors. By understanding and leveraging the gut-brain axis, researchers envision a future where neuroimmune-directed therapy becomes a cornerstone in liver cancer treatment.
As the scientific community eagerly awaits peer review and further validation of these findings, the implications of this research could pave the way for transformative advancements in the field of liver cancer therapy.
References:
- Bauer, K. C., Trehan, R., Ruf, B., Myojin, Y., Benmebarek, M.-R., Ma, C., Seifert, M., Nur, A., Qi, J., Huang, P., Soliman, M., Green, B. L., Wabitsch, S., Springer, D., Rodriguez-Matos, F. J., Ghabra, S., Gregory, S. N., Matta, J., Dawson, B., Golino, J., Xie, C., Dzutsev, A. K., Trinchieri, G., Korangy, F., & Greten, T. F. (2024). The Gut Microbiome Controls Liver Tumors via the Vagus Nerve. bioRxiv. https://doi.org/10.1101/2024.01.23.576951